


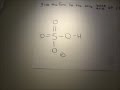



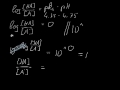








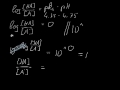
The conjugate acid of H P O 4 2 − is H 2 P O 4 − H 2 P O 4 − → H + + H P O 4 2 − The base and its conjugate acid differ by one proton only. The conjugate system involves proton transfer. The conjugate acid of H2PO4- is H3PO4 or phosphoric acid with a added proton. If it is conjugate acid, add a proton then remove a negative charge. If it is conjugate base, remove a proton then add a negative charge. H3PO4 is a tribasic acid, thus ionising in three steps.The conjugate base is formed when an acid loses its proton. Thus, HPO42- is the conjugate base of H2PO4- (which is an acid in step II, but is the conjugate base of H3PO4 in step I) The conjugate base of an acid, any acid, is defined as the acid "LESS" a proton, H^+. The conjugate acid of a base, any base, is defined as the base "PLUS" a proton. Phosphoric acid, H_3PO_4, is the parent acid. If it loses a proton, H^+, we conserve both mass and charge, and H_2PO_4^- results. And what is the conjugate base of this beasty? Similarly, what is the conjugate base of the Bronsted Lowry acid hpo4 2 -? Hence an acid donates a proton to form it's corresponding conjugate base while a base accepts a proton to form it's corresponding conjugate acid. From Bronsted Lowry theory , we can conclude that the conjugate base of HPO4^-2 is PO4^-3. Simply so, what is the conjugate acid of hpo2 − 4? Table of acids and their conjugate bases Well, to get the conjugate base we REMOVE a proton. And to get the conjugate acid we ADD a proton… And so…[math]H_{2}PO_{4}^{-} + H^+ → H_{3}PO_{4}[/math] And thus phosphoric acid is the conjugate acid of [math]H_{2}PO_{4}^{-}[/math] and the conju... H3PO4. Within the Brønsted–Lowry acid-base theory (protonic), a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton (hydrogen ion). The conjugate base of H2PO4... chemistry. The conjugate base of H 2 Which of the following are conjugate acid-base pairs: This question has multiple correct options. View solution. Of the following complex ions, one is a Bronsted-lowry acid. That one is: View solution. Conjugate Acid Of H2po4. Source(s): https://shrink.im/a0ffX. 0 0. Anonymous. 5 years ago. For the best answers, search on this site https://shorturl.im/8dmlP. congugate acid is H+ and conjugate base is PO4-0 0. Anonymous. 6 years ago. problematic subject. do a search on yahoo or google. just that can assist! 0 3. its an acid, H2PO4- is called dihydrogen phosphate ion. It is the conjugate base of Phosphoric Acid H3PO4 and the conjugate acid of monohydrogen phosphate ion HPO42
[index] [6566] [4883] [4651] [2799] [8006] [9191] [8107] [1290] [8012] [8813]
Determine acid/base ratio of a buffer The content of this video is designed to accompany the 12th edition of "Chemistry The Central Science" by Brown, Lemay, Bursten, Murphy, and Woodward. The ti... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Chemistry: Learn more at: http://www.pathwaystochemistry.com/chemistry-qa/videos/conjugate-acid-base-pairs/ Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together... Determine acid/base ratio of a buffer - Duration: 12:43. Peter Klappa 38,107 views. 12:43. Using the HH equation to calculate partial charges on amino acids - Duration: 5:26. ... Here I am just answering one of my hw problems. I will try to post as much hw as I can This is for an OCHEM CLASS. All the answers provided are checked by th... In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺)....
Copyright © 2024 m.esportics.site